Intelligent Design Home

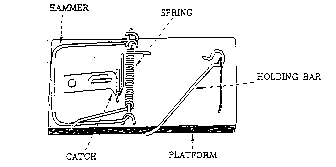
Figure 1. A household mousetrap. The working parts of the trap are labeled. If any of the parts are missing the trap does not function.
Source: http://www.bioanim.com/
Figure 1:
3d- image of a cilium cross-section
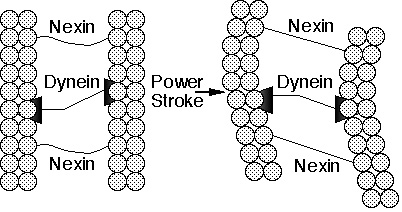
Figure 2. Schematic drawing of part of a cilium. The power stroke of the motor protein, dynein, attached to one microtubule, against subfiber B of a neighboring microtubule causes the fibers to slide past each other. The flexible linker protein, nexin, converts the sliding motion to a bending motion
Some bacteria boast a marvelous swimming device, the flagellum, which has no counterpart in more complex cells. In 1973 it was discovered that some bacteria swim by rotating their flagella. So the bacterial flagellum acts as a rotary propellor -- in contrast to the cilium, which acts more like an oar.
The bacterial flagellum is an example of what Michael Behe describes as an irreducibly complex system. In his book, Darwin's Black Box, he explains that such irreducibly complex systems could not have arisen by a gradual step-by-step Darwinian process.
Because the bacterial flagellum is necessarily composed of at least three parts -- a paddle,a rotor, and a motor -- it is irreducibly complex. Gradual evolution of the flagellum, like the cilium, therefore faces mammoth hurdles. (Behe, p. 72)
The structure of a flagellum is quite different from that of a cilium. The flagellum is a long, hairlike filament embedded in the cell membrane. The external filament consists of a single type of protein, called "flagellin." The flagellin filament is the paddle surface that contacts the the liquid during swimming. At the end of the flagellin filament near the surface of the cell, there is a bulge in the thickness of the flagellum. It is here that the filament attaches to the rotor drive. The attachment material is comprised of something called "hook protein." The filament of a bacterial flagellum, unlike a cilium, contains no motor protein; if it is broken off, the filament just floats stiffly in the water. Therefore the motor that rotates the filament-propellor must be located somewhere else. Experiments have demonstrated that it is located at the base of the flagellum, where electron microscopy shows several ring structures occur. The rotary nature of the flagellum has clear, unavoidable consequences ... (Behe, pp. 70-72)
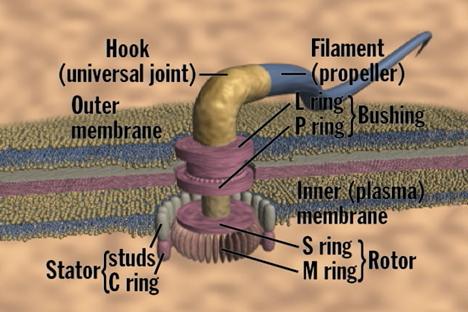
Copyright © 1998 Access Research Network. All rights reserved.
International copyright secured.
For an animated view of the flagellum in action,
click here)
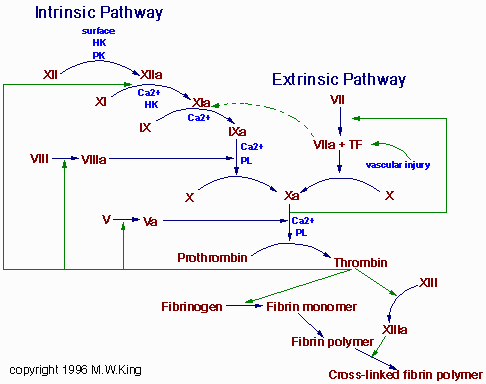
The blood clotting cascades represent incredible complexity for something most people take for granted and consider a simple process. However, this is not the case. Nothing other than an intricately designed mechanism for blood clotting could account for the marvelous operation of how our blood clots - and how clots are removed.
Consider the complexity of the following.
(Source:
http://www.indstate.edu/thcme/mwking/blood-coagulation.html)
The intrinsic cascade is initiated when contact is made between blood and exposed endothelial cell surfaces. The extrinsic pathway is initiated upon vascular injury which leads to exposure of tissue factor (TF) (also identified as factor III), a subendothelial cell-surface glycoprotein that binds phospholipid. The green dotted arrow represents a point of cross-over between the extrinsic and intrinsic pathways. The two pathways converge at the activation of factor X to Xa. Factor Xa has a role in the further activation of factor VII to VIIa as depicted by the green arrow. Active factor Xa hydrolyzes and activates prothrombin to thrombin. Thrombin can then activate factors XI, VIII and V furthering the cascade. Ultimately the role of thrombin is to convert fribrinogen to fibrin and to activate factor XIII to XIIIa. Factor XIIIa (also termed transglutaminase) cross-links fibrin polymers solidifying the clot. HK = high molecular weight kininogen. PK = prekallikrein.